正高级
Supervisor of Doctorate Candidates
Supervisor of Master's Candidates
Hits:
DOI number:10.1021/acs.jpcc.1c04351
Affiliation of Author(s):扬州大学化学化工学院
Teaching and Research Group:物理化学
Journal:The Journal of Physical Chemistry C
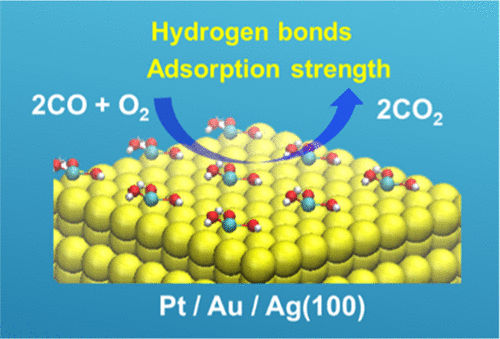
Abstract:In a recent experiment (Cao et al., Nature2019,565, 631), iron-based single-atom catalysts (SACs) deposited on platinum nanoparticles exhibited exceptionally high activity for CO oxidation. In this article, via first-principles calculations, we have discovered two key factors that can explain the extraordinary catalytic performance of SACs in the experiment: (i) formation of hydrogen bonds in the rate-determining step of catalysis and (ii) moderate adsorption strength of CO on the surface. The former factor facilitates the reaction by stabilizing the intermediates, while the latter makes the adsorbed reactant more reactive to the catalyst. Interestingly, the experimental coverage of SACs on the Pt surface achieved using atomic layer deposition techniques is actually the optimal one that favors both factors. Guided by these findings, we have explored the Fe1(OH)3 SAC on different metal supports for CO oxidation. Density functional theory and microkinetic simulations suggest that compared with the experimental catalyst on Pt(100), Fe1(OH)3 on Au(100) or Ag(100) shows an equal or even higher activity, and the catalysis follows a partially different mechanism where the rate-determining step corresponds to a later process. These results not only predict alternative highly active catalysts for CO oxidation but also provide design principles for novel and efficient SACs for other reactions.
First Author:Rufang Zhao
Indexed by:SCI
Correspondence Author:Yang WANG
Volume:125
Issue:29
Page Number:15987–15993
Translation or Not:no
Date of Publication:2021-07-19
Included Journals:SCI
Links to published journals:https://pubs.acs.org/doi/10.1021/acs.jpcc.1c04351