正高级
Supervisor of Doctorate Candidates
Supervisor of Master's Candidates
Hits:
DOI number:10.1021/acs.jcim.1c00735
Affiliation of Author(s):Yangzhou University
Teaching and Research Group:物理化学
Journal:Journal of Chemical Information and Modeling
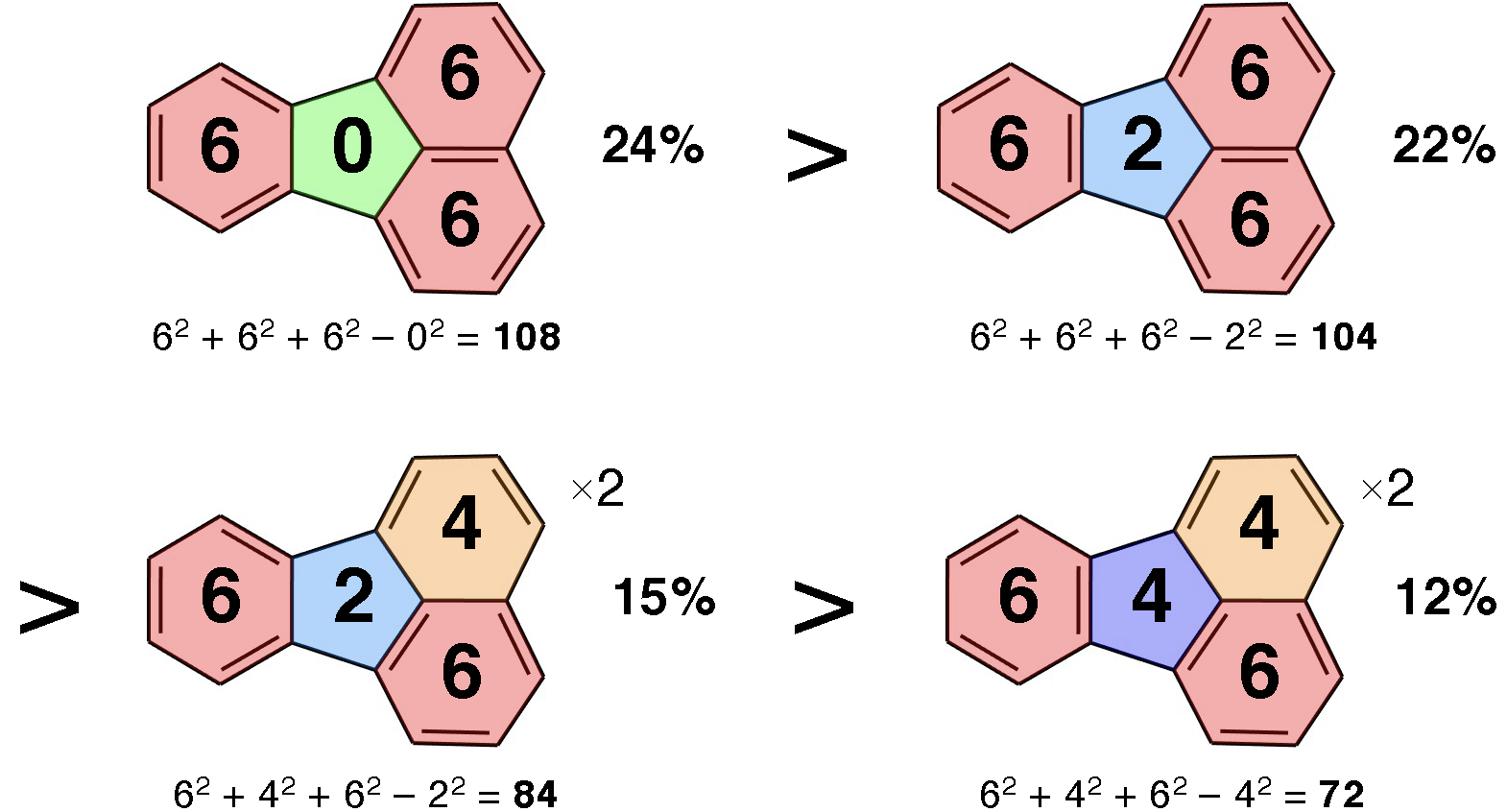
Abstract:The Fries rule is a simple, intuitive tool to predict the most dominant Kekulé structures of polycyclic aromatic hydrocarbons (PAHs), which is valuable for understanding the structure, stability, reactivity, and aromaticity of these conjugated compounds. However, it still remains an empirical hypothesis, with limited qualitative applications. Herein, we verify, generalize, and quantify the Fries rule based on the recently developed resonance analysis of the DFT wave functions of over 1500 PAH and fullerene molecules with over a billion Kekulé structures. The extended rules, counting the numbers of electrons within all rings (not just sextets), are able to rank the relative importance of all Kekulé structures for all considered systems. The statistically meaningful quantification also opens a way to evaluate ring aromaticity based on the resonance theory, which generally agrees well with conventional aromaticity descriptors. Furthermore, we propose a purely graph-based aromaticity indicator nicely applicable to PAHs and fullerenes, with no need of any quantum chemistry calculations, so that it can make valuable predictions for molecular properties that are related to local aromaticity.
First Author:Yang WANG
Indexed by:SCI
Volume:In press
Translation or Not:no
Date of Publication:2021-08-24
Included Journals:SCI